
What Is TMS? A Non-Invasive, FDA-Cleared Treatment for Depression
Discover how the FDA-cleared therapy works and how newer treatments like SAINT® can deliver care in a shorter timeframe

Transcranial Magnetic Stimulation (TMS) is a non-invasive, FDA-cleared therapy for neuropsychiatric and neurological conditions that uses gentle magnetic pulses to stimulate areas of the brain involved in mood regulation1, helping to restore healthier patterns of brain activity.
A brief history of FDA milestones
Over the past two decades, significant optimization of TMS technology has expanded its clinical utility. This evolution has led to a series of FDA clearances that reflect both growing evidence and increasingly precise treatment approaches. TMS was first cleared by the FDA in 2008 for the treatment of treatment-resistant major depressive disorder (MDD). This was followed by approval for cortical mapping in 2009 and the acute treatment of pain associated with migraine with aura in 2013. In 2017, TMS received FDA clearance for obsessive-compulsive disorder (OCD)2.
Major advancement
A significant leap forward occurred in 2022, when the FDA cleared the Stanford Accelerated Intelligent Neuromodulation Therapy System (SAINT®), a breakthrough accelerated TMS therapy for treatment-resistant MDD. SAINT is the first and only TMS treatment to use functional MRI (fMRI) to map a person’s brain connectivity and identify the optimal anatomical region for a condensed course of magnetic stimulation.3 This personalized approach enables more precise treatment delivery and a much shorter timeframe than conventional TMS. Specifically, SAINT combines an MRI-guided selection of the targeted brain region with an accelerated stimulation regimen involving multiple short TMS sessions every day for five days.4
According to Nolan Williams, M.D., director of the Stanford University Brain Stimulation Lab, which developed the SAINT protocol, this FDA clearance was groundbreaking for many reasons. In addition to being the first rapid-acting neuromodulation commercially available, SAINT also marked the first time radiology was formally integrated into the treatment of mental illness.5
“Because SAINT requires neuroimaging, this clearance marks the first official involvement of radiology in mental illness care,” he said. While imaging has long been used to support psychiatric diagnoses, this was the first FDA-cleared use of imaging to directly guide psychiatric treatment.
What treatment looks like
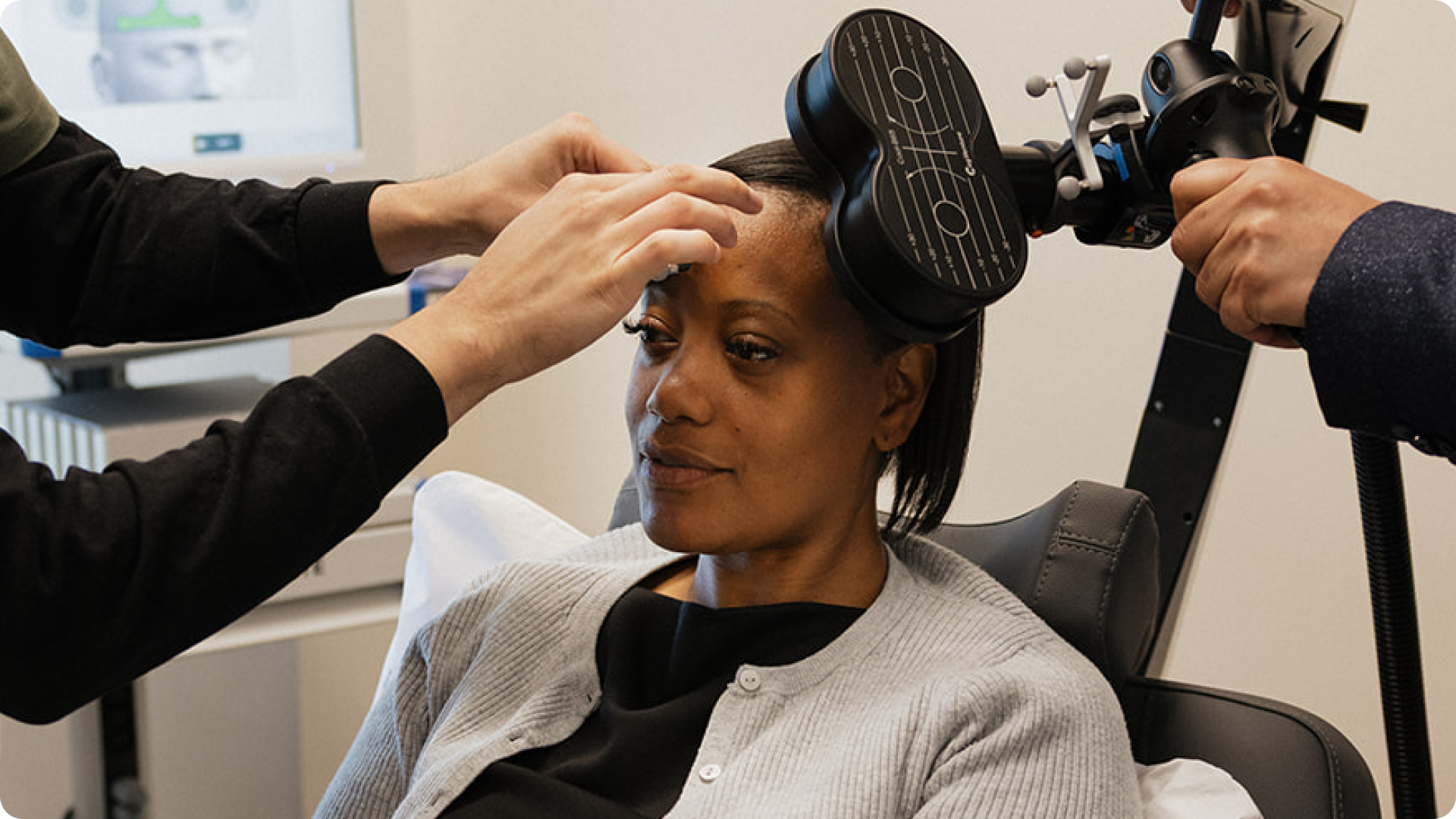
During a TMS session, a clinician places a magnetic coil gently against your scalp, typically over the left prefrontal cortex, the region strongly associated with mood regulation. As magnetic pulses are delivered, people typically feel a tapping sensation on the scalp as the targeted brain area is stimulated.
TMS therapies are considered very safe, even when used in combination with antidepressants. The most common side effects are mild scalp discomfort or temporary headaches during early sessions, which typically diminish as treatment continues.
Importantly, TMS is not electroconvulsive therapy (ECT). It does not produce seizures, does not require anesthesia, does not cause memory loss, and does not produce the systemic side effects often associated with certain medications.
Treatment options
Conventional TMS
- FDA-cleared in 2008 for treating treatment-resistant major depressive disorder
- FDA cleared in 2017 for obsessive-compulsive disorder
- Sessions last 20-40 minutes
- Five sessions per week
- Completed over 6+ weeks total
SAINT®
- FDA-cleared in 2022 for treatment-resistant major depressive disorder (MDD)
- Shorter sessions delivered 10 times per day with rest intervals
- Completed in just five days
- Designed for rapid symptom improvement through personalized targeting
Considering TMS as part of your care
If you are currently taking antidepressants and still searching for the relief you deserve, a form of TMS, including SAINT therapy, may be an excellent addition to your treatment plan. Ask your care provider for more information about TMS and its full range of offerings. To engage with a medical professional at Salma Health, click here.
1. https://pmc.ncbi.nlm.nih.gov/articles/PMC8864803/
2. https://pmc.ncbi.nlm.nih.gov/articles/PMC8864803/
3. https://bbrfoundation.org/content/fda-clears-saint-rapid-acting-brain-stimulation-approach-those-suffering-resistant-major
4. https://psychiatryonline.org/doi/full/10.1176/appi.pn.2022.10.10.40
5. https://psychiatryonline.org/doi/full/10.1176/appi.pn.2022.10.10.40
Why Salma Health?
With locations in La Jolla, Laguna Hills, and the Bay Area, Salma Health offers advanced mental and behavioral health care in California, with both in-person and virtual options. We support individuals living with depression, anxiety, PTSD, OCD, brain injuries, and related conditions, using personalized, science-backed approaches.
Start Your Journey Today
Getting started doesn’t have to be overwhelming. You can begin with a 15-minute Care Options Call, connect with our care team, complete a comprehensive intake, or schedule online. We meet you where you are and build care around your needs. Schedule your first appointment today and experience a higher standard of brain care—grounded in science, clarity, and continuity.

.avif)
.avif)
.avif)