.png)
What is SAINT®?
SAINT® is a groundbreaking, FDA-cleared form of MRI-guided neuromodulation for treatment-resistant major depressive disorder (TRMDD). Developed by doctors at Stanford University and available throughout the U.S., SAINT combines advanced brain imaging, personalized targeting and accelerated treatment protocols to deliver rapid relief for adults who have not responded to medications or therapy.
Also known as “SAINT® TMS”, Stanford Accelerated Intelligent Neuromodulation Therapy delivers 10 sessions of treatment per day for five days, with each session tailored to the individual’s brain activity. This accelerated approach allows many patients to achieve remission from their depression in days instead of months.
How does SAINT® TMS work?
SAINT uses structural and functional MRI scans to map each person’s unique brain activity. A proprietary algorithm identifies the most strongly connected region of the left dorsolateral prefontal cortex (DLPFC) in relation to the subgenual cingulate, a region known to be overactive in depression.
By delivering high dose theta-burst magnetic stimulation to this precise target, SAINT can reduce abnormal subgenual activity and restore healthier mood regulation. Treatments follow the principle of spaced learning, which includes short, focused periods of stimulation with rest intervals in between to maximize effectiveness.
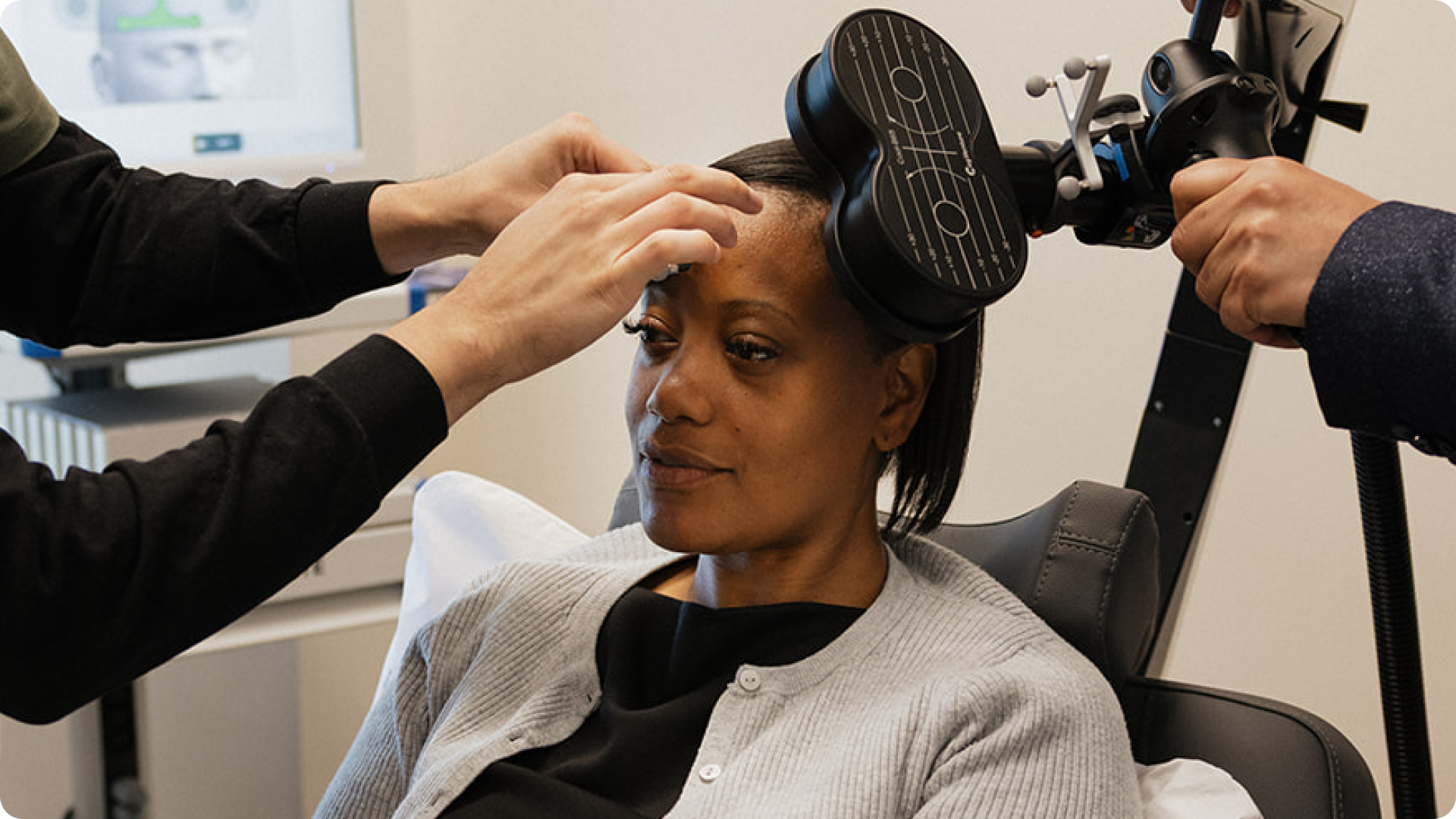
Treatment
Getting started with SAINT begins with a consultation with a psychiatrist at Salma Health. If the therapy is right for you, the next step is an MRI scan to map brain activity, which guides the creation of a personalized treatment plan. Many health insurance plans are covering SAINT on a case-by-case basis, please ask about this during your SAINT consult.
Pre-treatment: Baseline and Mapping
- Clinical and psychiatric assessments.
- MRI brain scans and targeting of the optimal stimulation site for SAINT
Day 1-5: Treatment Sessions
- 10 daily sessions (10 minutes each, with 50-minute breaks) delivered over five consecutive days.
- Delivered comfortably in a clinic setting, with private resting rooms during the 50-minute breaks.
- Patients remain awake and may resume normal activities afterward.
- Side effects include fatigue, headaches and a slight tapping feeling on the skull.
Day 5: Evaluation
- Post treatment assessment following the final round of therapy to measure symptom relief.
- Discharge planning and development of a long-term support plan.
Clinical results
In a double blind randomized controlled trial (RCT), 79% of patients achieved remission after SAINT treatment, compared to only 13% receiving sham treatment. Open label studies confirm 80-90% remission rates following the five-day treatment.
Getting started
Getting started with SAINT begins with a conversation. If you’re interested in exploring whether this treatment is right for you, the first step is a brief call with one of our providers. We’re here to guide you through the process and answer any questions along the way.




.png)
.png)
.png)